Types of clinical trials.

Variations of the classic parallel clinical trial design are described, such as crossover, sequential, or factorial clinical trials.
We’ve already talked at other times about the king of experimental designs, the randomized clinical trial, in which a population is randomly assigned into two groups to undergo the intervention under study, one of the groups, and to serve as a control group, the other one.
his is the most common side of the King, the parallel clinical trial, which is ideal for most studies about treatment, for many studies about prognosis or prevention strategies and, with its peculiarities, for studies assessing diagnostics tests. But the King is very versatile and has many other sides to accommodate to other situations.
Types of clinical trials
If we think about it for a moment, the ideal design would be one that allows us to test in the same individual the effect of the intervention study and of the stablished control (placebo or standard treatment) because parallel testing is an approach that assumes that both groups respond equally to both interventions, which is always a risk of bias that we try to minimize with randomization.
If we had a time machine we could test the intervention in all, note what happens, turn back in time and repeat the experiment with the control intervention. So, we could compare the two effects. The problem is, the more vigilant of you will have already guessed, that time machine has not been invented yet.
Cross-over clinical trials
But was has been already invented is the cross-over trial design, in which each subject acts as his own control.
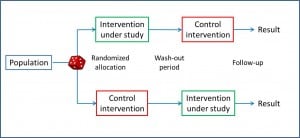
In this type of trial, every subject is randomized to a group, the corresponding intervention is performed, it takes place a washout period, and the other intervention is carried out. Although this solution is not as elegant as the time machine, the cross-over study defenders argue that the variability within each individual is less than the inter-individual variation.
Thus, the estimate may be more accurate that the obtained with a parallel trial and we usually require smaller sample sizes. However, before using this design, a number of considerations have to be done.
Logically, the effect of the first intervention should not cause irreversible changes or be very long, because it would affect the effect of the second. In addition, the washout period must be long enough to avoid leaving any residual effect of the first intervention.
We must also consider whether the order of the interventions could affect the final outcome, because in this case only results of the first intervention will be reliable (sequence effect). Another problem is that, by having a longer duration, patient characteristics may change during the study and may be different in the two periods (period effect).
And finally, be alert to the losses during follow-up, more frequent in longer studies and which have greatest impact in cross-over studies trials and with more repercussion in final results than in the case of parallel trials.
Factorial clinical trials
Imagine now that we want to test two interventions (A and B) in the same population. Can we do it with only one trial, saving costs of any kind?. Yes, we can. We only have to design a factorial clinical trial. In this type of trial, each participant undergoes two consecutive randomizations. She’s first assigned to the intervention A or placebo (P), and then, to the intervention B or placebo, with which we’ll have four study groups: AB, AP, BP and PP.
Obviously, the two interventions must act through independent mechanisms to be able to assess the results of the two effects independently.
It’s usually studied one more mature and plausible hypothesis and one that has been less tested, ensuring that the evaluation of the second doesn’t affect the inclusion and exclusion criteria of the first. Furthermore, it’s not desirable that any of the two interventions have many troublesome effects or be poorly tolerated, because the lack of compliance with one treatment will affect the compliance with the other.
In cases in which the two interventions seem not to be independent, their effect could be studied separately (AP vs. PP and BP vs. PP), but we’ll lost the advantages of the design and a larger sample size will be required.
Sequential clinical trials
Other times it may happen that we are in a hurry to finish the study soon. Imagine a very bad disease that kills people by dozens and we’re trying a new treatment. We’ll want to have it available as soon as possible (if it works, of course), so we’ll pause the trial and discuss its results after being tested the treatment in a certain number of participants, because if we can already show the usefulness of the treatment, we’ll end the study.
This is the type of design that characterizes the sequential clinical trial. Remember the in the parallel clinical trial the right thing is to pre-calculate the sample size. In this design, with a more Bayesian’s mentality, we stablish and statistic whose value determines an explicit ending rule, whereby the sample size depends on the previous observation of the study. When this statistic reaches the preset value we are confident enough to reject the null hypothesis and end the study.
The problem is that each stop and analysis increases the error of reject the null hypothesis being true (type 1 error), so it’s not recommended to perform many interim analysis. Moreover, the final analysis of results is more complex because we have to take into account the interim analysis. This type of trials is very helpful with very quick impact interventions, which is often seen in studies about dose titration of opioids, hypnotics, and poisons of that kind.
Clustered clinical trials
There are other occasions where individual randomization makes no sense. Think we have taught physicians of a health center a new technique to better inform their patients and want to compare it with the old one. We cannot say the same physician to inform some patients in a way and other patients in another, since there would be a strong possibility that the two interventions contaminate to each other. It would be more logical to teach a group of medical centers and not teach another group and compare the results.
Here we randomize health centers to form or not their doctor. This is the cluster allocations design. The problem with this design is that we have little assurance that participants of different groups behave independently, so the sample size required can be greatly increased if there is great variability among groups and little within each group.
In addition, we must perform and aggregate analysis of results, because if we do it individually confidence interval will be falsely narrowed and we can find false statistical significance. The usual practice is to calculate a weighted statistic for each group and make final comparisons with it.
Community clinical trials
The last of the series we are going to deal with is the community trial, in which the intervention is applied to populations. As it’s performed on populations under actual conditions it has high external validity and it often allow us recommending cost-effective measures based on their results.
The problem is that it is often difficult to establish a control group, it may be more difficult to determine the sample size needed and is more complex to perform causal inference from their results. It is the typical design for evaluating public health measures such as water fluoridation design, vaccinations, etc.
We’re leaving…
As you can see, the King has many sides. But it also has lower-rank relatives, but which are not less worthy. It’s so because it has a whole family of quasi-experimental studies consisting of trials that are not randomized or controlled, or any of both things. But that’s another story…

Como siempre muy claro e ilustrativo, esta vez más facilito y de contenido cultural.