Classification of epidemiological studies.

Classification of epidemiological studies is made according to their observational or analytical character, directionality and temporality and sampling.
As we already know from previous posts, the evidence-based medicine systematics begins with a knowledge gap that moves us to ask a structured clinical question. Once we have elaborated the question, we will use its components to make a bibliographic search and obtain the best available evidence to solve our doubt.
And here comes, perhaps, the most feared task of evidence-based medicine: the critical appraisal of the evidence found. Actually, the thing is not so much since, with a little practice, the critical reading consists only of systematically applying a series of questions about the article that we are analyzing. The problem sometimes comes in knowing what questions we have to ask, since this system has differences according to the design of the study that we are evaluating.
What is an epidemiological design?
Here, by design we understand the set of procedures, methods and techniques used with the study participants, during the data collection and during the analysis and interpretation of the results to obtain the conclusions of the study. And there are a myriad of possible study designs, especially in recent times when epidemiologists have been led to design mixed observational studies. In addition, the terminology can sometimes be confusing and use terms that do not clarify well what is the design we have in front of us. It’s like when we get to a wedding of someone from a large family and we meet a cousin we do not know where it comes from. Even if we look for physical similarities, we will most likely end up asking him: and you, which family you belong? Only then will we know if he belongs to the groom or to the bride.
What we are going to do in this post is something similar. We will try to establish a series of criteria for classifying studies to finally establish a series of questions whose answers allow us to identify which family they belong to.
Structured clinical question
To begin with, the type of clinical question to which the work tries to answer can give us some guidance. If the question is of diagnostic nature, it is most likely that we will be faced with what is called a diagnostic test study, which is usually a design in which a series of participants are subjected, in a systematic and independent way, to the test in study and to the reference pattern (the gold standard). It is a type of design especially made for this type of questions but do not just take it from me: sometimes we can see diagnostic questions that can be tried to be solved with other types of studies.
If the question is about treatment, it is most likely that we are facing a clinical trial or, sometimes, a systematic review of clinical trials. However, there are not always trials on everything we look for and we may have to settle for an observational study, such as a case-control or a cohort study.
In case of questions of prognosis and etiology/harm we may find ourselves reading a clinical trial, but the most usual thing is that it is not possible to carry out trials and we only have observational studies.
Characteristics of epidemiological studies
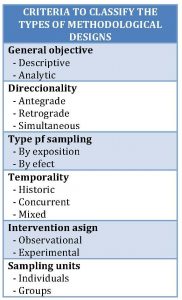
According to the objective, the studies can be descriptive or analytical. A descriptive study is one that, as the name suggests, only has the descriptive purpose of telling how things are, but without intending to establish causal relationships between the risk factor or exposure and the effect studied (a certain disease or health event, in most cases). These studies answer not very complex questions like how many? where? or to whom ?, so they are usually simple and they serve to elaborate hypotheses that later will need more complex studies for their demonstration.
By contrast, other analytical studies do try to establish such relationships, answering questions like why? how to deal with? or how to prevent? Logically, to establish such relationships it will need to have a group with which to compare (the control group). This will be a useful clue to distinguish between analytical and descriptive studies if we have any doubt: the presence of a comparison group will be typical of analytical studies.
The directionality of the study refers to the order in which the exposure and the effect of such exposure are investigated. The study will have an antegrade directionality when the exposure is studied before the effect and a retrograde directionality when the opposite is done. For example, if we want to investigate the effect of smoking on coronary mortality, we can take a set of smokers and see how many die of coronary diseases (antegrade) or, conversely, take a set of deaths from coronary heart disease and look to see how many smoked (retrograde). Logically, only studies with anterograde directionality can ensure that the exposure precedes the effect in time (I’m not saying that one is the cause of the other). Finally, to say that sometimes we can find studies in which exposure and effect are studied at the same time, talking then of simultaneous directionality.
The type of sampling has to do with how to select the study participants. These can be chosen because they are subject to the exposure factor that interests us, to having presented the effect or to a combination of the two or even other criteria other than exposure and effect.
Our fourth criterion is temporality, which refers to the relationship in time between the researcher and the exposure factor or the effect studied. A study will have a historical temporality when effect and exposure have already occurred when the study begins. On the other hand, when these events take place during the study, it will have a concurrent temporality. Sometimes the exposure can be historical and the effect concurrent, speaking then of mixed temporality.
Clarifying a pair of terms
Here I would like to make a point about two terms used by many authors and that will be more familiar to you: prospective and retrospective. Prospective studies would be those in which exposure and effect did not occur at the beginning of the study, while those in which the events have already occurred at the time of the study would be retrospective. To curl the curl, when both situations are combined we would talk about ambispective studies. The problem with these terms is that sometimes they are used indistinctly to express directionality or temporality, which are different terms. In addition, they are usually associated with specific designs: prospective with cohort studies and retrospective with case and control studies. It may be better to use the specific criteria of directionality and temporality, which express the aspects of the design more precisely.
Two other terms related to temporality are those of transversal and longitudinal studies. Transversals are those that provide us with a snapshot of how things are at a given moment, so they do not allow us to establish temporal or causal relationships. They tend to be prevalence studies and always of a descriptive nature.
On the other hand, in longitudinal studies variables are measured over a period of time, so they do allow establishing temporary relationships, but the researcher dos not control how the exposure is assigned to participants. These may have an antegrade (as in cohort studies) or retrograde (as in case and control studies) directionality.
The penultimate of the six criteria that we are going to take into account is the assignment of the study factors. In this sense, a study will be observational when the researchers are mere observers who do not act on the assignment of the exposure factors. In these cases, the relationship between exposure and effect may be affected by other factors, known as confusion, so they do not allow drawing conclusions about causality. On the other hand, when the researcher assigns the effect in a controlled manner according to a previous established protocol, we will talk about experimental or intervention studies. These experimental studies with randomization are the only ones that allow establishing cause-effect relationships and are, by definition, analytical studies.
The last of the criteria refers to the study units. The studies can be carried out on individual participants or on population groups. The latter are ecological studies and community trials, which have specific design characteristics.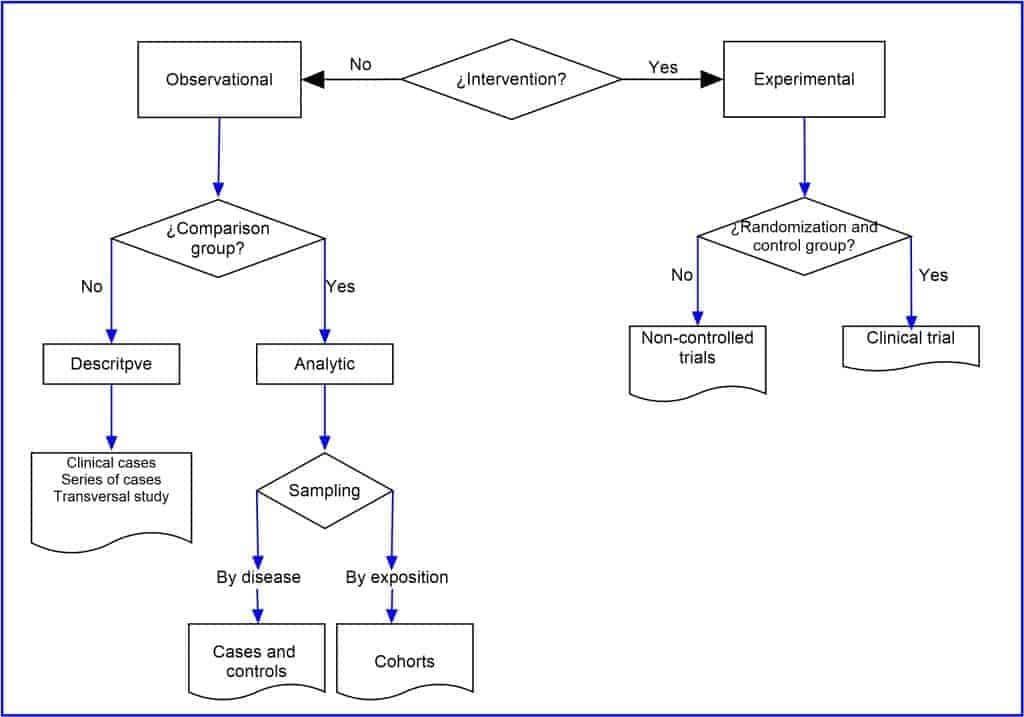
If the observational study is analytical, we will look at the type of sampling, which may be due by disease or study effect (case-control study) or by exposure to the risk or protection factor (cohort study).
Finally, if the study is experimental, we will look for if the exposure or intervention has been assigned randomly and with a comparison group. In the affirmative case, we will find ourselves in front of a randomized controlled clinical trial. If not, it is probably an uncontrolled trial or another type of quasi-experimental design.
We’re leaving…
And here we will stop for today. We have seen how to identify the most common types of methodological designs. But there are many more. Some with a very specific purpose and their own design, such as economic studies. And others that combine characteristics of basic designs, such as case-cohort studies or nested studies. But that is another story…
