Number remaining at risk.

Today we are going to do an act of social justice.
At least from the point of view of analyzing the results of clinical trials.
You will wonder what the post of today is about. Well, everyone knows that when we try a new treatment for a disease, the first thing we want to know is how many patients we cure, for example, of that disease, or how many we prevent from dying from the disease.
Imagine that, along with 99 other individuals, you participate in a clinical trial. Ultimately, 2 deaths are prevented with the treatment, so the number needed to treat (NNT) will be 50. And this is the main outcome of the trial, a NNT = 50.
Surely, the two survivors will be more than happy, but among the rest of the participants a clamor of 98 voices will rise asking: and me? What’s up with me? What about me?
Our act of social justice, which I was referring to at the beginning, has to do with these 98 participants.
Risk measures
We all know the different measures of association and impact that we can use in a clinical trial. The first to be considered is the risk ratio (RR), which is the ratio of presenting the event between the treated (Rt) and the controls (Rc). With this measure we calibrate the protective or favorable effect that the intervention has on the result.
We can also calculate the risk reductions between the two groups. The relative risk reduction (RRR) would be the decrease in risk in the intervention group compared to the risk observed in the controls. On the other hand, the absolute risk reduction (ARR) indicates the difference in risk between the two groups.
The number needed to treat (NNT)
Finally, the NNT is the most widely used impact measure and arguably the one with the greatest clinical value, since it represents the effort required to achieve a specific clinical benefit, either avoiding an adverse event or achieving a beneficial one.
In addition to measuring the efficiency of the intervention, the NNT has many other advantages, such as implicitly incorporating the baseline risk without treatment and RRR, but giving a more objective idea of the effect. We already know that the effect always seems greater if we only assess the RRR.
In addition, the NNT helps us to make a more objective assessment without being misled by clinical factors such as the form of presentation of the disease or the severity of the result we are measuring.
But not everything are advantages. It turns out that the NNT is a somewhat selfish indicator and does not care about the fate of those patients that are not reflected in its value. What happens, for example, with those who are not prevented from dying by treatment? What is the risk of dying for those who do not contribute to the value of the NNT?
Number remaining at risk (NRR)
To answer this question, another indicator has been devised that is added to the entire family of risk, association and impact measures of clinical trials: the number remaining at risk (NRR).
The NRR deals with what the NNT forgets, since it estimates the average prognosis of presenting the result of interest among those treated, once those who achieve it thanks to the treatment have been excluded. The formula to calculate it would be the following:
NRR = Rt / (Rt-Rc)
If you look closely, this formula could be written as Rt x 1 / (Rt-Rc).
Rt-Rc is the ARR, and its inverse is the NNT. Thus, we can calculate the NRR in the following way:
NRR = Rt x NNT.
Let’s see some examples
I think that, in order to better understand the usefulness of the NRR, we are going to look at the results of several trials carried out with different treatments with which we pursue our usual effort to prevent death from that terrible disease that is fildulastrosis.
We are going to test three drugs called, let’s not think too much, A, B and C. We compare them against a placebo, look at the number of deaths at the end of the trial and use Calcupedev calculator to get the risk and impact measures. You can see the results of the three studies in the attached table.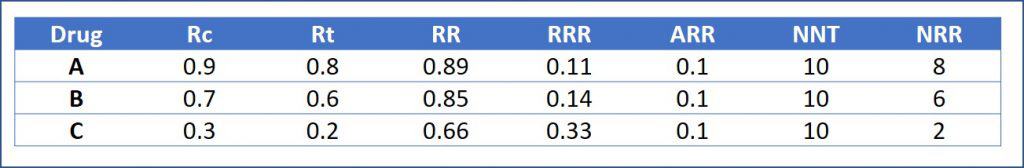
As you can see, the mortality figures are different in the three trials, although in all of them the ARR of mortality with treatment is 0.1 (10%). Therefore, the NNT that we obtain is the same in the three studies, 10. This means, as we already know, that we avoid one death from fildulastrosis for every 10 patients we treat.
And here we would stay if we did not give ourselves to think about what happens to those who do not avoid death thanks to treatment. We could even simplify and say that the three treatments have similar efficacy.
To avoid this, let’s look at the NRRs, which are different in the three studies. Looking at the results, we quickly understand that the prognosis is radically different in the patients in the three trials. For example, in the trial with drug A, we prevented one death for every 10 treated, but 8 of the remaining 9 died despite receiving the treatment. In drug C group, the one with the best prognosis, we have to treat 10 equally to prevent one death from fildulastrosis, but only 2 of the other 9 die.
Looking at these differences in prognosis in the three studies, we could no longer so happily conclude that all three treatments have similar efficacy because we get the same NNT.
The number remaining at risk turned out to be an odds
Let’s take one more twist to understand the meaning of the NRR even better. And for this, we are going to think about its relationship with RR and RRR.
We can calculate NRR based on RR using the following formula:
NRR = RR / (1-RR)
If you look at the quotient of the previous formula, in the numerator we have a probability (RR) and in the denominator its complement (1-RR). And what is the probability that an event occurs divided by the probability that it does not occur (its complement)? You got it, an odds.
If we understand RR as the probability of an event occurring in response to treatment, we can understand NRR as the odds of that event occurring versus preventing it. For example, an NRR of 5 would mean that the patient is 5 times more likely to die from the disease (despite treatment) than to avoid death thanks to treatment.
Furthermore, as the NRR can be expressed as a function of the RR, and since both the RR and the RRR are calculated from the risks in treated and controls, it can be mathematically demonstrated that each RRR is associated with a certain NRR, regardless of the RAR or NNT values. Thus, an RRR of 0.1 is associated with an NRR of 9, an RRR of 0.2 with an NRR of 4, an RRR of 0.5 with an NRR of 1… Curiosities of the numbers.
The NRR may even have a value lower than 1, which will indicate that it is more likely to have a beneficial effect from the treatment than an adverse one. Of course, for this assumption to occur, the RRR has to be large (> 50%), the rate of adverse events must be very low, or both conditions must be met.
Conclusion
We have seen in this post the usefulness of the NRR, although the logical thing is not to use it in isolation, but as a complement to the NNT, with which we can more accurately assess the benefits-risks of the intervention.
The NRR will indicate the absolute number of patients who will present the event of interest and will serve us, as we have seen, to explain discordant results between trials with different results that may be due to prognostic differences between participants, which may present more or less aggressive presentations of disease.
The latter may induce us to use the NRR as a measure of relative efficacy between various treatments, but it is not an indicator that has been designed for this purpose, so we must avoid falling into this temptation.
We are leaving…
And here we are going to leave it for today. We have seen how the NRR is an indicator that is easy to calculate and understand by most clinicians. Anyway, do not get carried away by enthusiasm and use it for anything. For example, formal studies with cost-effectiveness analysis decision techniques require more complex statistical models, beyond the reach of most clinicians and only available to privileged minds. But that is another story…
